Hello science fans,
We had the chance to do a project about the competition of two species, Lemna minor and Chlamydomonas reinhardtii, for nitrate!
Nitrate,
or NO3-, is known to be essential in the proteins production process
of many species, especially species undergoing photosynthesis . It
exists in the majority of ecosystems and is consumed by their hosts. We
are going to present you two species which supply themselves with
nitrate and which we chose for our final biosensors project.
Lemna minor,
called “water lentil” or “duckweed” in common language, have air sacs
in their leaves, allowing them to float at the surface of ponds water.
Curiously, during winter and low temperatures, their leaves eject the
air and they sink at the bottom of water.
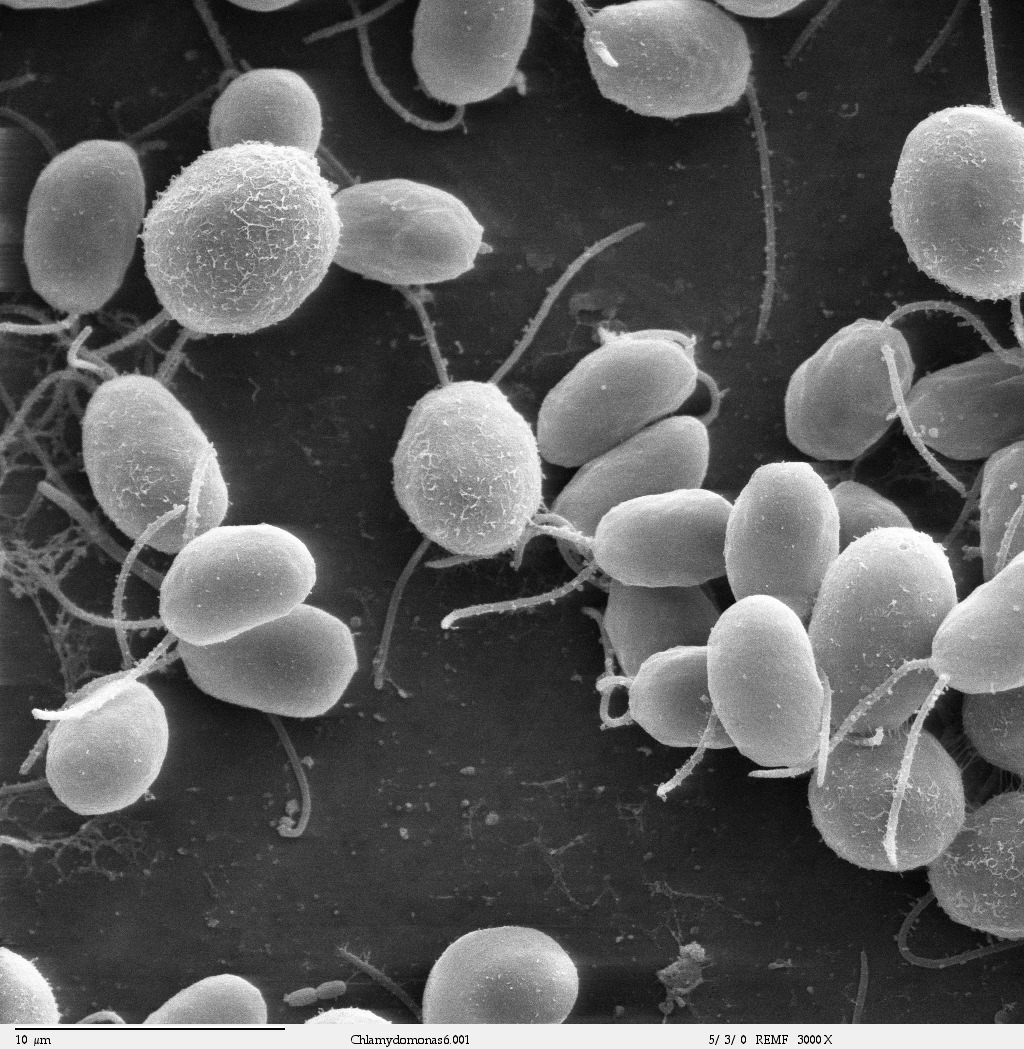 |
| Chlamydomonas reinhardtii, wikipedia commons. |
Chlamydomonas reinhardtii
are green algae living in a similar environnement. They have the
capacity to move thanks to its flagella or to lose them in some
conditions. Moreover, their regeneration time is relatively quick for
an organism, which is an interesting characteristic to follow in
research. Usually, in aquapony water lentils are introduced in aquarium
to fight against algae invasion. In fact, this species is consuming
faster the nitrate, dying algae by asphyx. By this way, we chose to see to what extent the amount of nitrate in water influences Chlamydomonas reinhardtii growth, in case of Lemna minor presence.
How did we make to answer this question ~
To
study this characteristic, we followed a simple protocol, showed below.
We chose to test 3 different concentrations of nitrate in water: 0, 120
and 240 milligram per liter. This means that the 0 corresponds in fact
to the natural nitrate concentration of tap water, which was at 15
milligram per liter.
For each concentration, there were three different types of beakers: beakers containing only Lemna minor, or only Chlamydomonas reinhardtii,
or both species. Each organism was introduced in the same initial
quantity in each beaker and we placed the beakers at the same place, in a
greenhouse in order to have similar experimental conditions. This
allowed us to limit variability between our samples due to external
factors. From this, we were able to distinguish the nitrate influence
and the species interaction if there is, by comparing beakers results
between them. We took in account several parameters: the light, pH,
temperature, C. reinhardtii concentration, and L. minor
growth, all overtime. The first three ones were to control the
environment of our organisms and the last one, to follow how the
experiment changes.
In
total, we had 27 beakers to follow: we chose to have 3 beakers per type
of beakers and per concentration. This is called “replicates” and it is
particularly important in research to have significant results.
All of our days could be summarized as taking measurements of pH, light, L. minor surface and biomass, concentration of C. reinhardtii.
Respectively, we took data with pH paper, luxmeter, pictures & the
software imageJ, a scale and finally, a microscope with slides made to
count. For the last day, we measured the final nitrate concentration of
each beaker in order to compare it with the concentration that was
originally in the water! We used a nitrate test which gave us a colour
corresponding to a concentration. We chose to use a spectrophotometer to
determine the colour absorbance and made a scale with knew
concentrations. From this and the absorbances of our samples, we were
able to determine their final nitrate concentration. :)
What did we find after the experiment?
Following
graphs don’t correspond to the final graphs with all replicates, maybe
too hard to read and so little much interesting, but it gives us the
trend obtained. The graph above is showing the concentration of nitrate
that we found in beakers at the end of the experiment. We can notice
that there is an aberration: globally, final concentration is higher
than the initial concentration. Moreover, it seems that more nitrate
there is in solution initially, faster the nitrate is consumed.
We suggest that it is due to the fact that the nitrate test was not accurate enough and maybe the fact that the spectrophotometer was saturated,without taking the real absorbance of the solution and so given a ‘false’ value of the final concentration in nitrate.
We suggest that it is due to the fact that the nitrate test was not accurate enough and maybe the fact that the spectrophotometer was saturated,without taking the real absorbance of the solution and so given a ‘false’ value of the final concentration in nitrate.
Both
graphs showed after represent the amount of both organisms for
different concentrations of nitrates. We can see a small difference
between concentrations, but unfortunately it is insignificant to
conclude on the nitrate influence on Lemna minor and Chlamydomonas reinhardtii.
To
go further, we have now a better idea of how this type of project is
going and the next point could be to do it again with a nitrate probe.
:)
Thank you for your reading, if you want to know more:
- You can read our report and all our documentation on our GitHub
- You should look at our Twitter and of course, our superb storify
- You can contact us by mail
- You can visualize our manipulation by watching this video
Lots of love,
Louise Dagher, Corentin Mathé--Deletang and Simon Fr


No comments:
Post a Comment